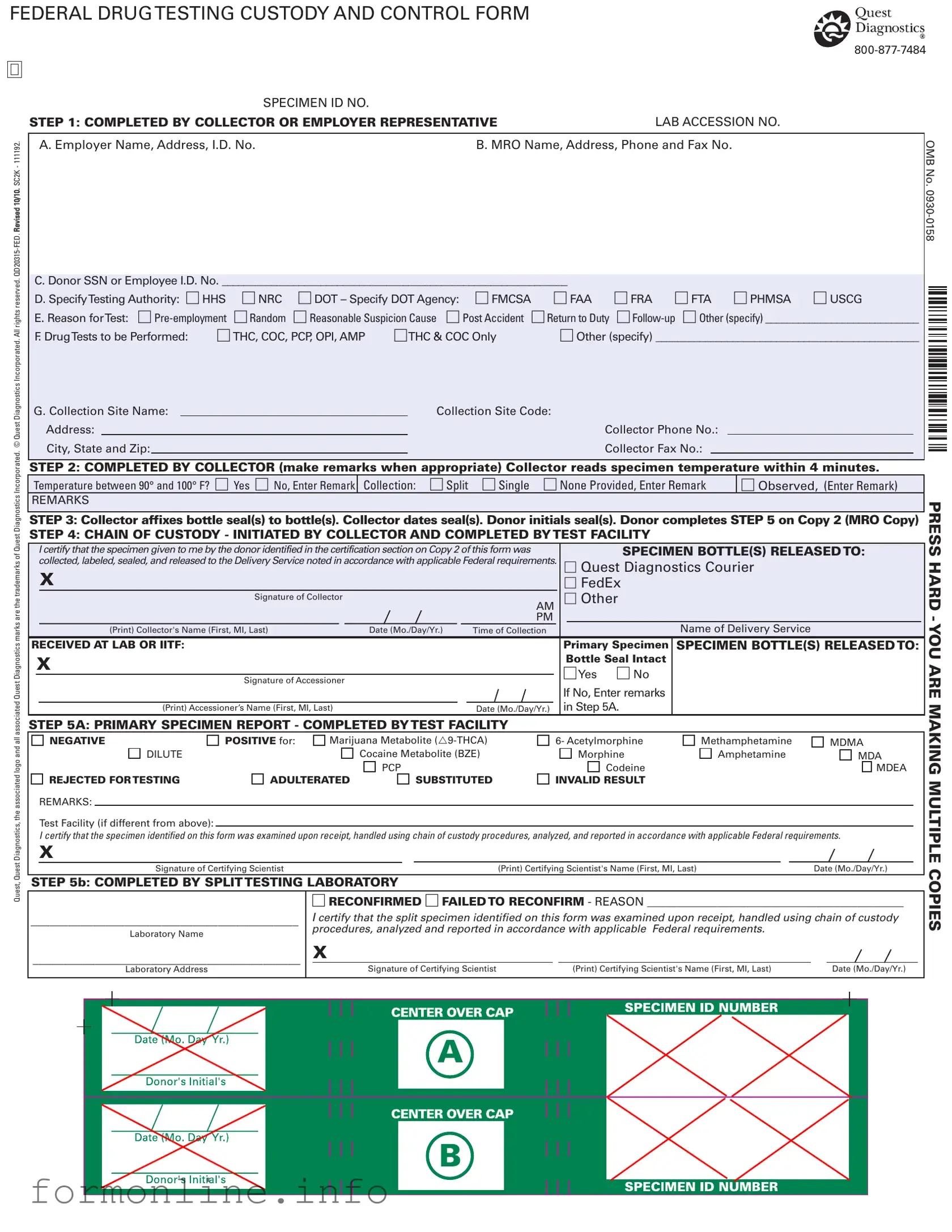
Fill Out a Valid Drug Screen Form
The Drug Screen form, officially known as the Federal Drug Testing Custody and Control Form, is a crucial document used in the drug testing process for various purposes, including pre-employment screenings and random tests. This form serves multiple stakeholders, including employers, collectors, and testing facilities, ensuring that the entire process adheres to federal regulations. Key elements of the form include sections for the employer's and medical review officer's (MRO) information, as well as the donor's identification, which may be a Social Security number or employee ID. The form also specifies the testing authority, such as the Department of Transportation (DOT) or the Department of Health and Human Services (HHS), and outlines the reasons for testing, which can range from random checks to post-accident evaluations. Furthermore, it details the types of drug tests to be performed, ensuring clarity on substances like THC, cocaine, and amphetamines. The form also includes a chain of custody section, which is vital for maintaining the integrity of the specimen throughout the testing process. With steps for collectors to follow, including temperature checks and sealing procedures, the Drug Screen form is designed to uphold the highest standards of accuracy and reliability in drug testing.
Common mistakes
-
Incomplete Information: One common mistake is failing to fill out all required fields. Each section, such as the employer name and donor information, must be complete. Omissions can lead to delays or even rejection of the test.
-
Incorrect Identification Numbers: Providing the wrong Social Security Number or Employee ID can create confusion. It is crucial to double-check these numbers for accuracy to ensure proper identification.
-
Misunderstanding Testing Authority: Selecting the wrong testing authority can have significant consequences. Ensure that the correct agency is specified, whether it’s HHS, NRC, or DOT, and be clear about which DOT agency applies.
-
Failure to Specify Reason for Testing: Not indicating the reason for the drug test can lead to misunderstandings. Whether it’s for pre-employment, random testing, or another reason, clarity is essential.
-
Neglecting Temperature Checks: The temperature of the specimen must be recorded within four minutes. If the temperature falls outside the acceptable range, it should be noted. This step is critical for maintaining the integrity of the test.
-
Ignoring Chain of Custody Procedures: Properly documenting the chain of custody is vital. Any lapses in this process can compromise the test results. Ensure that all signatures and timestamps are correctly filled out to maintain accountability.
Preview - Drug Screen Form
FEDERAL DRUG TESTING CUSTODY AND CONTROL FORM
SPECIMEN ID NO. |
|
STEP 1: COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE |
LAB ACCESSION NO. |
Quest, Quest Diagnostics, the associated logo and all associated Quest Diagnostics marks are the trademarks of Quest Diagnostics Incorporated. © Quest Diagnostics Incorporated. All rights reserved.
A. Employer Name, Address, I.D. No. |
|
|
B. MRO Name, Address, Phone and Fax No. |
||||||||||
|
|
|
|
|
|
|
|
|
|||||
C. Donor SSN or Employee I.D. No. _______________________________________________________________ |
|
|
|
|
|||||||||
D. SpecifyTesting Authority: HHS |
NRC |
DOT – Specify DOT Agency: FMCSA |
FAA |
FRA FTA PHMSA USCG |
|||||||||
E. Reason forTest: |
Random |
Reasonable Suspicion Cause Post Accident |
Return to Duty |
|
|||||||||
F. DrugTests to be Performed: |
THC, COC, PCP, OPI, AMP |
THC & COC Only |
Other (specify) ________________________________________________ |
||||||||||
G. Collection Site Name: |
|
|
|
|
|
Collection Site Code: |
|
|
|
|
|||
Address: |
|
|
|
|
|
|
Collector Phone No.: |
|
|
||||
City, State and Zip: |
|
|
|
|
|
Collector Fax No.: |
|||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
STEP 2: COMPLETED BY COLLECTOR (make remarks when appropriate) Collector reads specimen temperature within 4 minutes.
Temperature between 90° and 100° F? Yes No, Enter Remark |
Collection: Split Single None Provided, Enter Remark |
Observed, (Enter Remark) |
REMARKS
STEP 3: Collector affixes bottle seal(s) to bottle(s). Collector dates seal(s). Donor initials seal(s). Donor completes STEP 5 on Copy 2 (MRO Copy)
STEP 4: CHAIN OF CUSTODY - INITIATED BY COLLECTOR AND COMPLETED BY TEST FACILITY
|
I certify that the specimen given to me by the donor identified in the certification section on Copy 2 of this form was |
|
SPECIMEN BOTTLE(S) RELEASED TO: |
|||||||
|
collected, labeled, sealed, and released to the Delivery Service noted in accordance with applicable Federal requirements. |
Quest Diagnostics Courier |
||||||||
|
|
X |
|
|
|
|
|
FedEx |
||
|
|
Signature of Collector |
|
|
|
|
|
Other |
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
AM |
|
|
|
|
|
|
|
|
|
|
PM |
|
|
|
|
|
|
(Print) Collector's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|
Time of Collection |
|
|
Name of Delivery Service |
||
RECEIVED AT LAB OR IITF: |
|
|
|
|
|
Primary Specimen |
SPECIMEN BOTTLE(S) RELEASED TO: |
|||
|
X |
|
|
|
|
|
Bottle Seal Intact |
|
||
|
|
|
|
|
|
Yes No |
|
|||
|
|
Signature of Accessioner |
|
|
|
|
|
|
||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
If No, Enter remarks |
|
|
|
|
|
|
|
|
|
|
in Step 5A. |
|
|
|
|
(Print) Accessioner’s Name (First, MI, Last) |
|
|
|
Date (Mo./Day/Yr.) |
|
|||
STEP 5A: PRIMARY SPECIMEN REPORT - COMPLETED BY TEST FACILITY
NEGATIVE |
POSITIVE for: |
Marijuana Metabolite ( |
6- Acetylmorphine |
Methamphetamine |
MDMA |
|
DILUTE |
|
|
Cocaine Metabolite (BZE) |
Morphine |
Amphetamine |
MDA |
|
|
|
PCP |
Codeine |
|
MDEA |
REJECTED FOR TESTING |
ADULTERATED |
SUBSTITUTED |
INVALID RESULT |
|
|
|
REMARKS:
Test Facility (if different from above):
I certify that the specimen identified on this form was examined upon receipt, handled using chain of custody procedures, analyzed, and reported in accordance with applicable Federal requirements.
X
Signature of Certifying Scientist |
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
STEP 5b: COMPLETED BY SPLIT TESTING LABORATORY
RECONFIRMED FAILED TO RECONFIRM - REASON ____________________________________________
___________________________________________ |
I certify that the split specimen identified on this form was examined upon receipt, handled using chain of custody |
||||||||||||||||||||||||||||
procedures, analyzed and reported in accordance with applicable Federal requirements. |
|
|
|
|
|
||||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Name |
|
|
|
|
|
|||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||
___________________________________________ |
X |
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|||||||||||||
|
Signature of Certifying Scientist |
|
|
|
(Print) Certifying Scientist's Name (First, MI, Last) |
Date (Mo./Day/Yr.) |
|||||||||||||||||||||||
|
|
|
|
|
|
|
Laboratory Address |
|
|
|
|
||||||||||||||||||
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
|
OMB No.
PRESS HARD - YOU ARE MAKING MULTIPLE COPIES
Other PDF Templates
How to Get Employment Verification Letter - Individuals under 18 require authorization from a parent or guardian.
Patient Demographic Form PDF - Track the patient's age for medical and treatment purposes.
The process of obtaining the Arizona Board of Nursing License form can be streamlined by referring to resources such as AZ Forms Online, which provide essential guidance and support to ensure that applicants understand all requirements and can navigate the licensure process effectively.
Va 10-2850a - The VA 10-2850a allows applicants to outline their work history and relevant skills pertinent to VA employment.
Documents used along the form
When conducting drug screenings, several forms and documents may accompany the Drug Screen form. Each of these documents serves a specific purpose in ensuring compliance and maintaining accurate records throughout the testing process.
- Chain of Custody Form: This document tracks the handling of the specimen from the time it is collected until it is analyzed. It ensures that the specimen has not been tampered with and maintains the integrity of the testing process.
- Consent Form: This form is signed by the donor to give permission for the drug test to be conducted. It outlines the rights of the donor and the procedures involved in the testing process.
- Laboratory Analysis Report: After the specimen is tested, this report details the results of the analysis. It indicates whether the specimen tested positive or negative for specific substances and may include additional notes on the findings.
- Medical Review Officer (MRO) Report: The MRO evaluates the laboratory results and may contact the donor for further information. This report provides a summary of the MRO's findings and any necessary follow-up actions.
- RV Bill of Sale Form: For those buying or selling a recreational vehicle, the Texas RV Bill of Sale form is essential. It serves as a legal document that confirms the transaction and details the specifics of the exchange, providing necessary proof for registration and tax purposes. You can find the form at https://autobillofsaleform.com/rv-bill-of-sale-form/texas-rv-bill-of-sale-form.
- Employee Training Records: These documents show that employees have been trained on the drug testing policies and procedures. Maintaining these records is essential for compliance with regulations and company policies.
- Incident Report: If the drug test is conducted due to a specific incident, this report provides context. It details the circumstances surrounding the incident that led to the testing, such as an accident or safety violation.
Understanding these documents can help ensure a smooth drug testing process and maintain compliance with applicable regulations. Keeping accurate records is crucial for both employers and employees involved in drug screening procedures.
Similar forms
The Drug Screen form shares similarities with the Medical Release Form, which is often used in healthcare settings. Both documents require the identification of the individual undergoing testing or treatment, including personal details such as name and identification number. Additionally, they both necessitate the collection of consent from the individual to release their medical information. This ensures compliance with privacy laws, allowing healthcare providers to share relevant information with authorized parties while maintaining patient confidentiality.
In the context of mobile home transactions, parties involved should carefully consider the importance of having a formalized agreement to ensure clarity and protection for both the seller and the buyer. One essential document for this process is the Mobile Home Bill of Sale, which serves as a legal record of the sale and details various aspects of the ownership transfer, making it a crucial part of any mobile home purchase or sale.
Another document akin to the Drug Screen form is the Chain of Custody form used in criminal investigations. Like the Drug Screen form, the Chain of Custody form tracks the handling of evidence, ensuring that it has not been tampered with or altered. Both forms emphasize the importance of maintaining a clear and documented chain of custody. This process is crucial for validating the integrity of the evidence or specimen, which is essential in both legal and medical contexts.
The Employee Health Questionnaire is also similar to the Drug Screen form. This document gathers essential health information from employees, often for pre-employment screenings. Just as the Drug Screen form collects specific reasons for testing, the Employee Health Questionnaire asks about medical history and current health conditions. Both documents play a vital role in assessing the fitness of an individual for their job responsibilities while ensuring compliance with occupational health standards.
The Consent to Test form is another related document. It explicitly obtains permission from the individual to undergo drug testing, similar to how the Drug Screen form outlines the reasons and authority for the test. Both documents require the individual’s signature, indicating that they understand and agree to the testing process. This consent is crucial in protecting the rights of individuals while ensuring that employers can maintain a safe and drug-free workplace.
The Incident Report form is comparable as well, especially in workplace settings. This form documents specific incidents that may require drug testing, such as accidents or safety violations. Both the Incident Report and the Drug Screen form aim to provide a clear account of events leading to testing. By documenting these occurrences, organizations can ensure that appropriate measures are taken to address safety concerns and regulatory compliance.
Additionally, the Laboratory Test Requisition form bears resemblance to the Drug Screen form. This document is used to request various laboratory tests, including drug screenings. Both forms require detailed information about the individual being tested, including identification and the specific tests requested. They both facilitate communication between healthcare providers and laboratories, ensuring that accurate and timely testing occurs.
Lastly, the Pre-Employment Screening Form has similarities to the Drug Screen form. This form collects various types of information from potential employees, including drug testing requirements. Both documents are used to evaluate candidates before hiring, ensuring that they meet the necessary health and safety standards. They help employers make informed decisions while protecting the workplace environment.
Dos and Don'ts
When filling out the Drug Screen form, it is crucial to ensure accuracy and compliance. Here are four essential dos and don'ts to keep in mind:
- Do: Provide accurate personal information, including your name, address, and identification number.
- Do: Specify the reason for the test clearly, as this can impact the testing process and results.
- Don't: Leave any sections blank. Every part of the form must be completed to avoid delays.
- Don't: Alter or tamper with the form in any way, as this can lead to serious consequences.
Key takeaways
When it comes to filling out and using the Drug Screen form, there are several important points to keep in mind. Here are key takeaways that can help ensure the process runs smoothly:
- Accurate Information: Ensure that all fields are filled out completely and accurately. This includes the employer's name, address, and ID number, as well as the donor's Social Security number or employee ID.
- Testing Authority: Clearly specify the testing authority. This could be HHS, NRC, or DOT, among others. If DOT is selected, make sure to indicate the specific agency involved.
- Reason for Testing: Provide a clear reason for the test. Options include pre-employment, random, reasonable suspicion, and others. This helps in maintaining transparency in the testing process.
- Collection Site Details: Include the collection site name, address, and contact information. This is essential for any follow-up questions or concerns regarding the testing process.
- Temperature Check: The collector should read the specimen temperature within four minutes of collection. A temperature between 90° and 100° F is required for the sample to be considered valid.
- Chain of Custody: Maintain a clear chain of custody throughout the process. This includes signing off on the specimen and ensuring it is sealed properly to avoid any contamination or tampering.
- Documentation: Make sure to document any remarks or observations during the collection process. This information can be crucial for resolving any issues that may arise later.
By following these guidelines, both collectors and donors can contribute to a more effective and reliable drug screening process.
How to Use Drug Screen
Filling out the Drug Screen form accurately is essential for ensuring compliance with testing protocols. After you complete the form, it will be submitted for processing. Follow these steps carefully to ensure all necessary information is included.
- Start with the section labeled "COMPLETED BY COLLECTOR OR EMPLOYER REPRESENTATIVE." Fill in the Employer Name, Address, and I.D. Number.
- Provide the MRO Name, Address, Phone, and Fax Number.
- Enter the Donor's Social Security Number or Employee I.D. Number.
- Specify the Testing Authority by checking the appropriate box: HHS, NRC, or DOT. If DOT, indicate the specific agency (FMCSA, FAA, FRA, FTA, PHMSA, USCG).
- State the Reason for Test by selecting one from the options provided, such as Pre-employment or Random. If "Other," specify the reason.
- List the Drug Tests to be Performed. Choose from the options or specify others if needed.
- Fill in the Collection Site Name, Collection Site Code, and Address. Include the Collector's Phone Number, City, State, and Zip Code, along with the Collector's Fax Number.
- For the Collector section, check the specimen temperature within 4 minutes. Confirm if the temperature is between 90° and 100° F by marking Yes or No. If No, provide remarks.
- Indicate whether the collection was Split, Single, or None Provided. Add remarks if necessary.
- Affix the bottle seal(s) to the specimen bottle(s) and date them. Donor should initial the seal(s) and complete STEP 5 on Copy 2 (MRO Copy).
- Complete the Chain of Custody section. Certify that the specimen was collected, labeled, sealed, and released according to Federal requirements. Sign and print the Collector's Name, Date, and Time of Collection.
- In the RECEIVED AT LAB OR IITF section, confirm if the bottle seal is intact. If not, enter remarks in Step 5A. Sign and print the Accessioner’s Name and Date.
- In STEP 5A, indicate if the result is NEGATIVE or POSITIVE for specific substances. If rejected for testing, note the reason. Sign and print the Certifying Scientist's Name and Date.
- In STEP 5B, if applicable, indicate if the split specimen was reconfirmed or failed to reconfirm. Provide the reason if it failed. Sign and print the Certifying Scientist's Name and Date.